1. Current Research Topics
1. Current Research Topics
Chemistry solutions to PFAS pollution
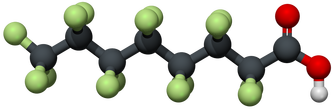
The high stability of C-F bonds in PFAS requires innovative and practical technologies both for the remediation of already contaminated environments and for the prevention of future pollution by perfluorinated chemicals.
We are working on different routes for solving this challenge. Research opportunities are available now (see the "Positions" tab for details).
For more information about PFAS pollution (we do not endorse the contents and viewpoints):
We are working on different routes for solving this challenge. Research opportunities are available now (see the "Positions" tab for details).
For more information about PFAS pollution (we do not endorse the contents and viewpoints):
- The Intercept Series: The Teflon Toxin (linked) and Bad Chemistry (linked)
- ACS Publications 2017 Virtual Issue: Poly- and Perfluoroalkyl Substances (linked)
Catalytic reduction of oxyanions in water
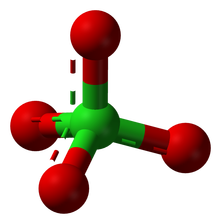
Perchlorate is a widespread and toxic anion in groundwater. Recently, discussions of perchlorate on Mars have also been renewed. For example:
However, most chemical reduction technologies developed thus far still have one or more of the following limitations: (1) low activity, (2) exotic reducing agents, (3) incompatibility with water, and (4) poor stability or longevity.
Based on the knowledge gained from previous studies on oxorhenium complex-based catalysts (see Publications), we aim to develop new biomimetic transition metal complexes and heterogeneous catalysts with the following features: (1) high activity, (2) high robustness, (3) easy preparation, and (4) low cost.
Our research involves a suite of intriguing directions such as (1) design, synthesis, and characterization of transition metal complexes with desired structure and activity, (2) characterization of metal species in heterogeneous support for aqueous phase reactions, (3) rational re-design for continuous improvement of activity, stability, and longevity of catalyst to be integrated with existing water treatment processes. We are strongly motivated by the unprecedented catalyst performance in 100% aqueous environment, the exciting discoveries in catalytic process control, and the unexpected new chemistry of the coordination metal complexes.
- Perchlorate on Mars: A chemical hazard and a resource for humans (linked)
- Perchlorates on Mars enhance the bacteriocidal effects of UV light (linked)
However, most chemical reduction technologies developed thus far still have one or more of the following limitations: (1) low activity, (2) exotic reducing agents, (3) incompatibility with water, and (4) poor stability or longevity.
Based on the knowledge gained from previous studies on oxorhenium complex-based catalysts (see Publications), we aim to develop new biomimetic transition metal complexes and heterogeneous catalysts with the following features: (1) high activity, (2) high robustness, (3) easy preparation, and (4) low cost.
Our research involves a suite of intriguing directions such as (1) design, synthesis, and characterization of transition metal complexes with desired structure and activity, (2) characterization of metal species in heterogeneous support for aqueous phase reactions, (3) rational re-design for continuous improvement of activity, stability, and longevity of catalyst to be integrated with existing water treatment processes. We are strongly motivated by the unprecedented catalyst performance in 100% aqueous environment, the exciting discoveries in catalytic process control, and the unexpected new chemistry of the coordination metal complexes.
2. Research Equipment (by Feb 2017, to be updated with new photos)
|
Glovebox : for handling hygroscopic or air-sensitive chemicals
New fumehood installed for synthetic works
Ion chromatography system for a variety of anions
|
Glovebag: for handling aqueous and air-sensitive samples
A portion of NMR spectrometers at UCR Chemistry Department
HPLC equipped with UV-vis and conductivity detectors
|












